C1.7.2.2 Analysis of Triprotic Phosphoric Acid by Titration
Couldn't load pickup availability
Analysis of Triprotic Phosphoric Acid by Titration
Phosphoric acid is a triprotic acid. When dissolved in water, it first gives up one proton and dissociates to dihydrogen phosphate, i.e. it reacts like a monoprotic acid (see formula 1). The addition of sodium hydroxide, e.g. during titration, first leads to complete dissociation of the phosphoric acid into dihydrogen phosphate.
The second protolysis, i.e. the reaction to hydrogen phosphate (see formula 2), occurs only after a high pH value has been reached, approx. pH 9. The third protolysis requires a considerably higher pH value (see formula 3). In experiment C1.7.2.2, the first two protolysis steps of phosphoric acid are determined in an automatic titration. 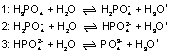
Components of experiments:
| 1 | 524 013 | Sensor-CASSY 2 |
| 1 | 524 220 | CASSY Lab 2 |
| 1 | 524 0672 | pH adapter S |
| 1 | 667 4172 | pH sensor with plastic shaft, BNC |
| 1 | 524 074 | Timer S |
| 1 | 337 4681 | Drop counter |
| 1 | 607 105 | Magnetic stirrer mini |
| 2 | 664 103 | Beaker, DURAN, 250 ml, squat |
| 1 | 665 997 | Graduated pipette, 10 ml |
| 1 | 666 003 | Pipetting ball (Peleus ball) |
| 1 | 665 847 | Burette, clear glass, 50 ml |
| 1 | 665 816 | Burette filling funnel, plastic, 25 mm diam. |
| 1 | 666 559 | Burette clamp for 1 burette, roller clamp |
| 1 | 300 02 | Stand base, V-shaped, small |
| 1 | 300 43 | Stand rod, 75 cm, 12 mm diam. |
| 1 | 300 11 | Saddle base |
| 1 | 301 26 | Stand rod, 25 cm, 10 mm diam. |
| 2 | 301 09 | Bosshead S |
| 2 | 666 555 | Universal clamp, 0...80 mm |
| 1 | 661 082 | Stopcock grease, 60 g |
| 1 | 674 3440 | Phosphoric acid, 10 %, 100 ml |
| 1 | 673 8421 | Sodium hydroxide solution, 1 mol/l, 1 l |
| 1 | 674 4640 | Buffer solution pH 4.00, 250 ml |
| 1 | 674 4670 | Buffer solution pH 7.00, 250 ml |
| 1 | 675 1600 | * Thymolphthalein solution, 0.1 %, 50 ml |
| 1 | additionally required: PC with Windows 7 or higher |
Articles marked with * are not INCLUDED, we do however recommend them to carry out the experiment.
Experiment Instructions Click Here










