C1.1.1.1 Determination of the Relative Atomic Mass of Metals
Regular price
$4,399.00
Couldn't load pickup availability
Determination of the Relative Atomic Mass of Metals
In experiment C1.1.1.1, the molar mass of some base metals will be determined. In order to do so, those metals - magnesium, for example - will be reacted with acids.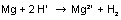 Different metals of the same weight produce different quantities of hydrogen. When using the same quantity of material, the same quantities of hydrogen are produced. This way, the relative molar mass of the respective metals can be determined.
Different metals of the same weight produce different quantities of hydrogen. When using the same quantity of material, the same quantities of hydrogen are produced. This way, the relative molar mass of the respective metals can be determined.
Components:
| 1 | 664 097 | Stoichiometric reaction vessel |
| 1 | 665 914 | Gas syringe, 100 ml with 3-way stopcock |
| 1 | 665 936 | Immersion tube manometer, after Schiele |
| 1 | 664 352 | Topping-up reservoir, 250 ml |
| 1 | 667 194 | Silicone tubing, 7 mm diam., 1 m |
| 1 | 382 21 | Stirring thermometer, -10...+110 °C |
| 1 | 667 312 | Glass connector, 2 x GL 18 |
| 1 | 667 017 | Scissors, 125 mm, round-ended |
| 1 | 667 027 | Tweezers, blunt, 130 mm |
| 1 | 664 131 | Beaker, Boro 3.3, 400 ml, squat |
| 1 | 665 753 | Measuring cylinder, 50 ml, with plastic base |
| 2 | 666 4659 | Adhesive magnetic board, 500 mm |
| 1 | 666 4662 | Holder, magnetic, size 2, 11...14 mm |
| 3 | 666 4665 | Holder, magnetic, size 5, 30...32 mm |
| 1 | 666 425 | Panel frame C50, two-level, for CPS |
| 1 | ADANBL124E | Analytical balance 120 g; 0,0001g, with USB interface |
| 1 | 674 6810 | Hydrochloric acid, 10 %, 1 l |
| 1 | 673 1000 | Magnesium, ribbon, 25 g |
| 1 | 661 081 | Aluminium, foil, 1 roll |
| 1 | 671 2000 | Calcium, granules, 25 g |
EXPERIMENT INSTRUCTIONS CLICK HERE
https://youtu.be/DCUpG2VGuls







